The Prosigna assay, designed from the foundations of breast cancer biology, provides the confidence to help make the right choices.
Knowing and being confident in the measurement of recurrence risk is an important consideration for deciding which treatment to recommend in HR+/HER2- breast cancer.1

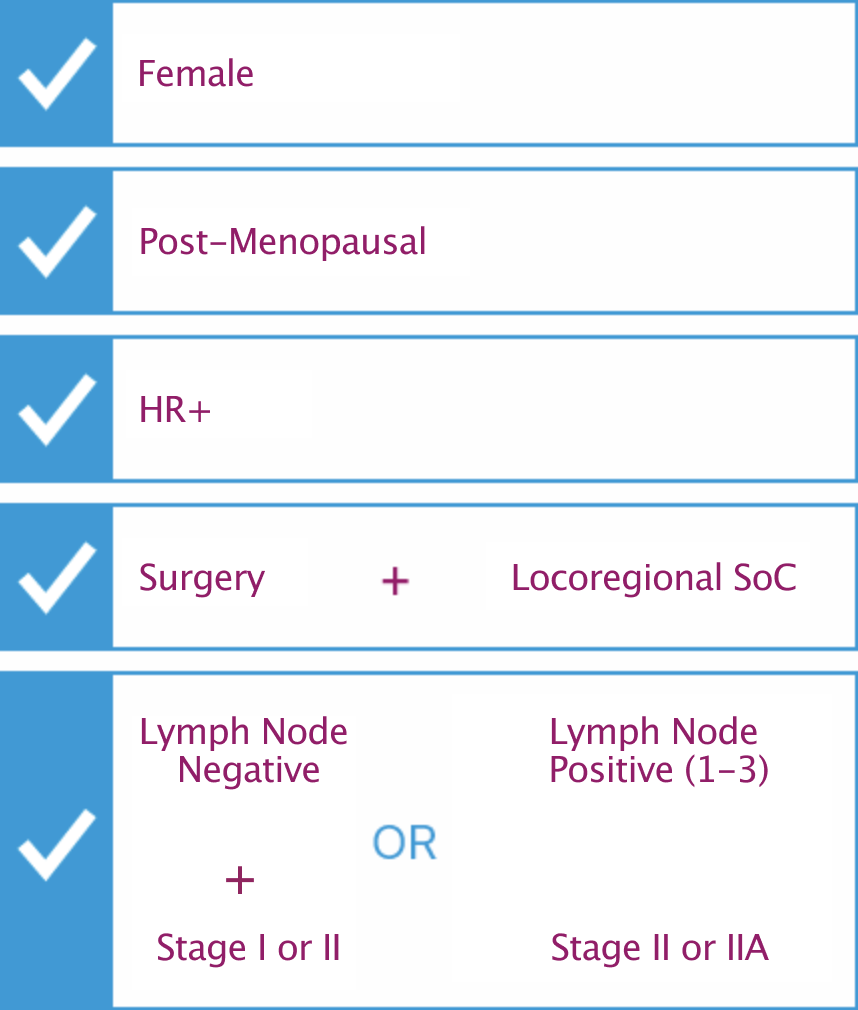
The Prosigna Breast Cancer Assay is intended for post-menopausal women with early-stage HR+ and HER2- breast cancer.
The Prosigna prognostic gene signature assay is a 2nd generation test that has shown improved performance when compared with older genomic assays.
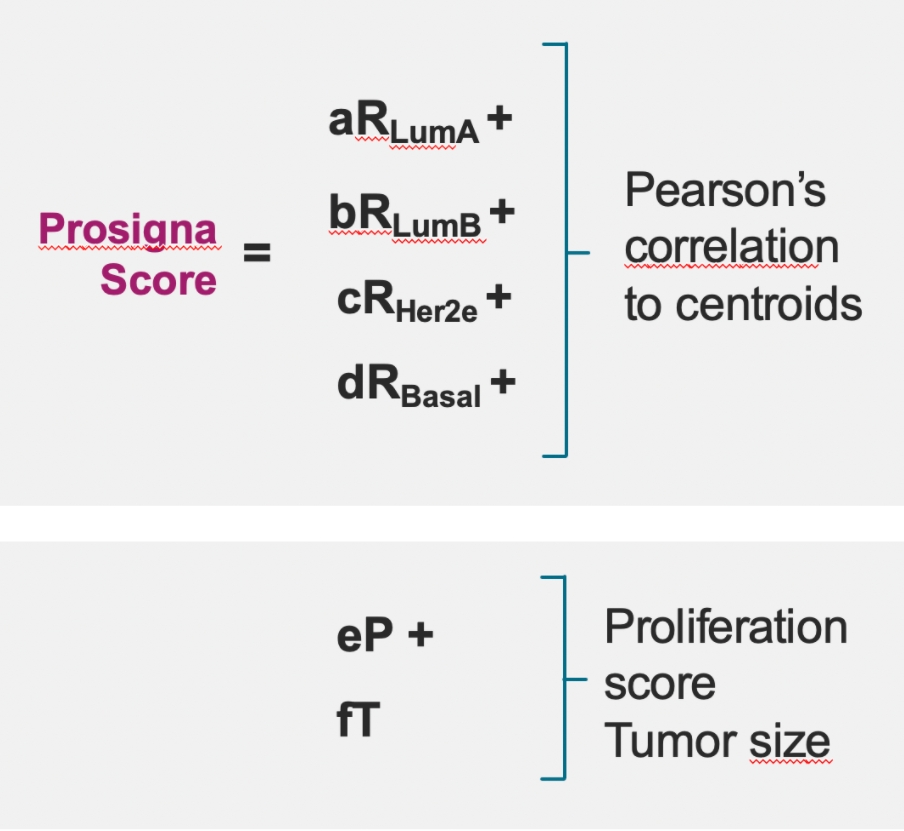
The Prosigna risk of recurrence (ROR) score adds significant prognostic information to conventional risk factors such as pathological analysis of the patient tumor, and protein markers detected using immunohistochemistry.2
Prosigna ROR score provides more accurate prognostic information on the risk of distant recurrence among gene-expression and immunohistochemical biomarkers.2
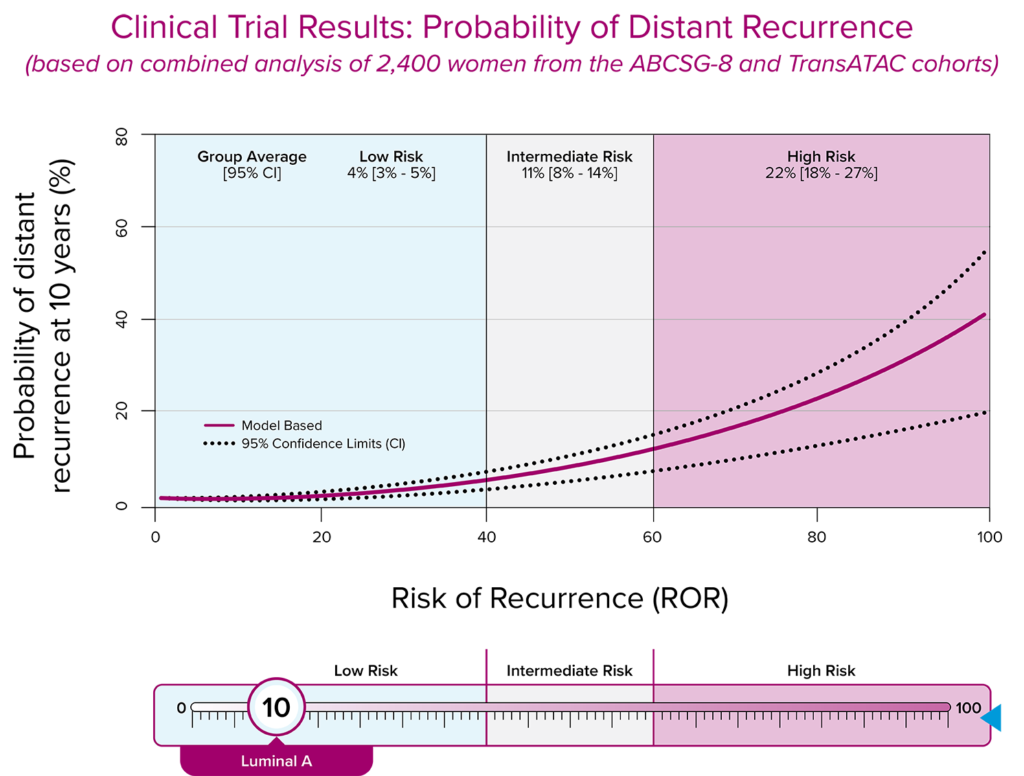
The Prosigna assay accurately classifies patients into low, intermediate, or high risk of distant recurrence.
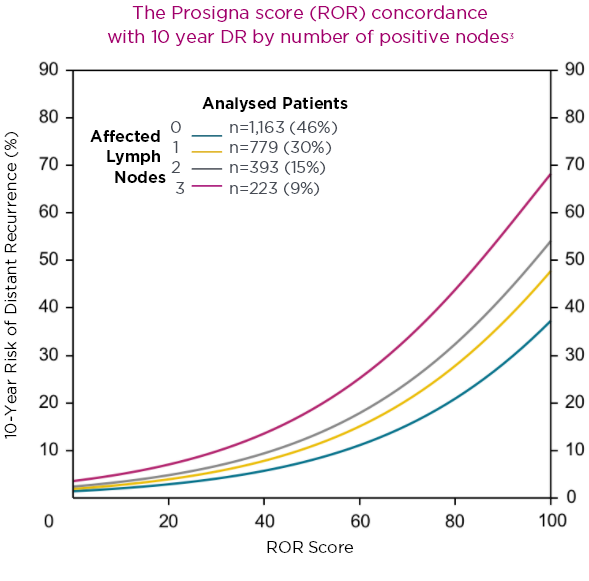
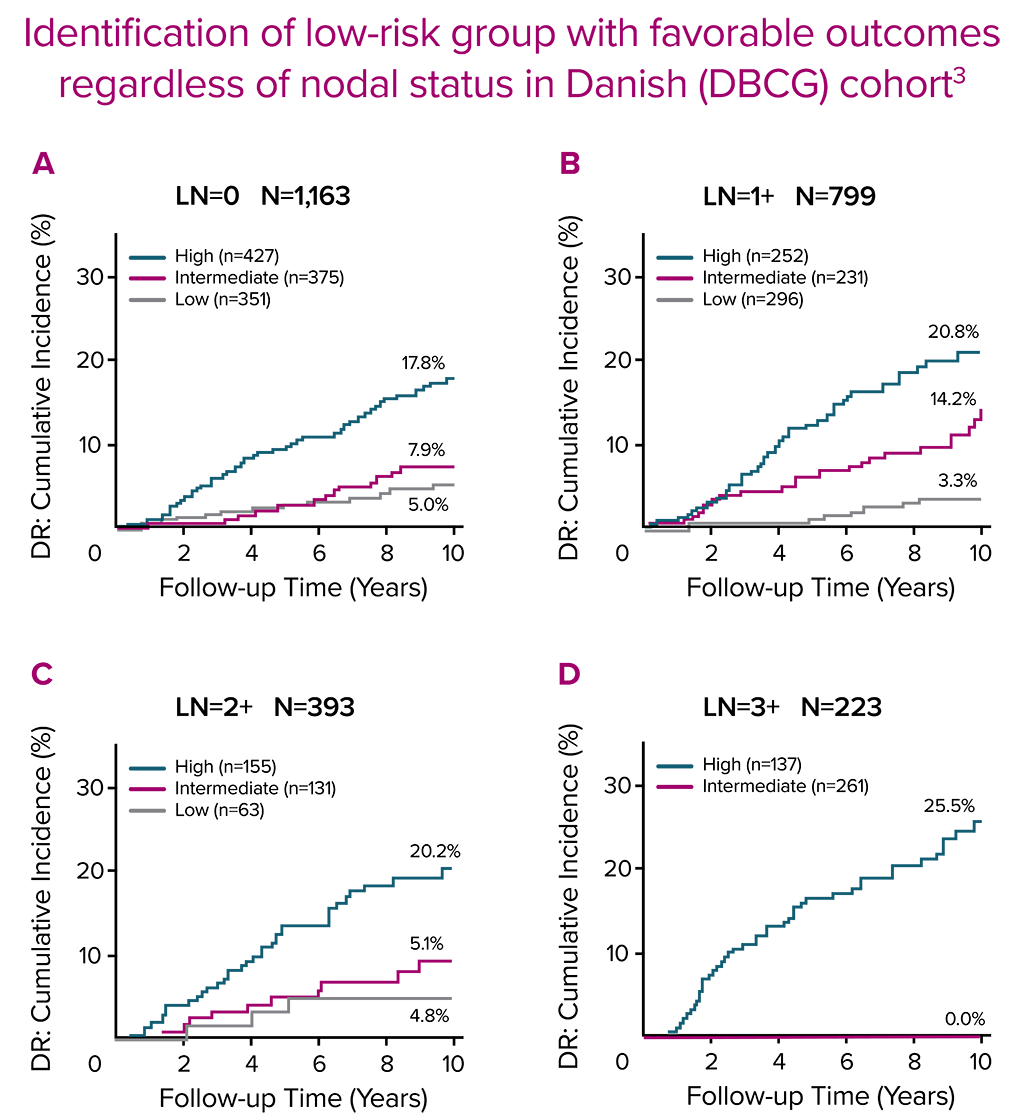
In a real-world setting, the Prosigna assay reliably identified low risk patients that were either node-negative or had one to three positive nodes, which could help clinicians decide if a patients could safely avoid adjuvant chemotherapy.3 The Danish Breast Cancer Cooperative Group (DBCG) examined distant recurrences in a comprehensive nationwide cohort consisting of 2,558 postmenopausal women with hormone receptor-positive early breast cancer treated with 5 years of endocrine therapy alone.3
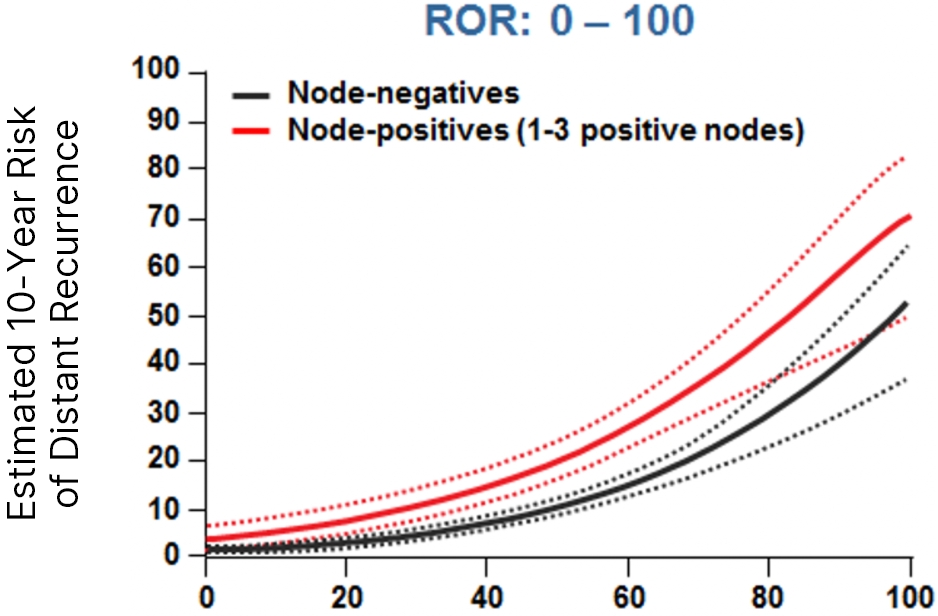
The Prosigna ROR score more accurately estimated the probability of 10-year distant recurrence in this population compared with the risk stratification provided by other gene panels.3.4
An accurate prognosis is the foundation of long-term treatment recommendations
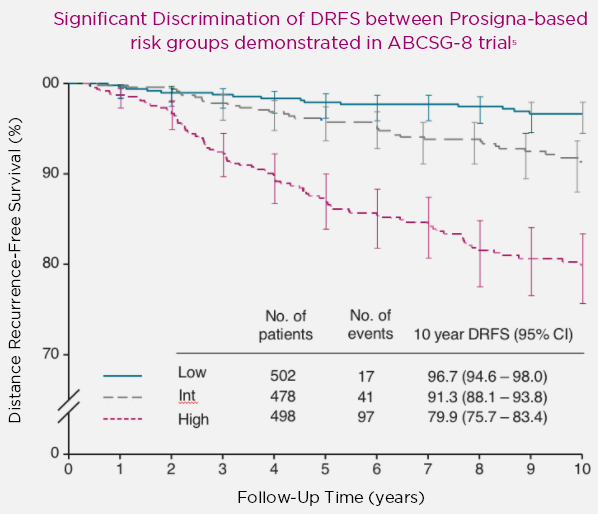
Extensive global evidence of clinical validity and clinical utility in over 5,200 patients treated with 5 years of standard-of-care hormone therapy and 10 year follow-up (prospective and populational)
Low and high risk differentiation is a key factor for determining a confident treatment decision
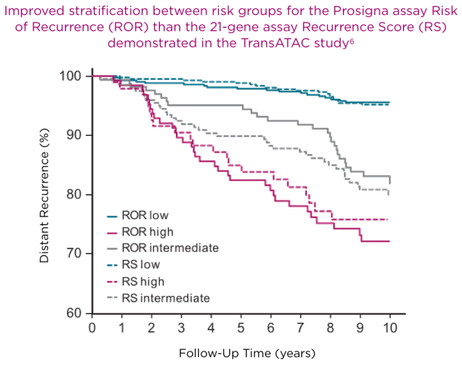
The recurrence risk results of more than a half (59%) patients in sub-analysis of 663 of the original 774 samples within the TransATAC study were discordant between Prosigna and Oncotype Dx. The observed outcomes were more consistent with the Prosigna risk category, not the Oncotype DX risk category.6
The Prosigna assay provides accurate prognostic information
Identify patients that can safely avoid over-treatment
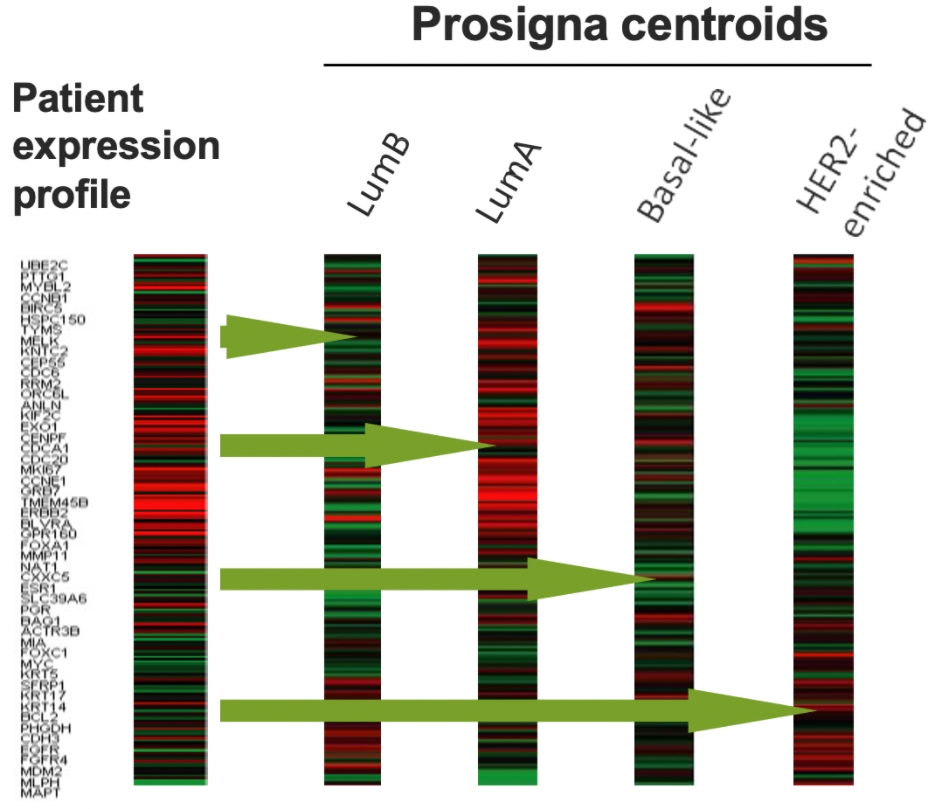
The Prosigna gene expression assay is the only breast cancer prognostic test developed from the four PAM50 molecular subtypes.9
The classification into the intrinsic subtypes, enables a more accurate assessment of tumor gene expression patterns.10

There is considerable discordance between IHC-based subtype and the Prosigna molecular subtypes.10
Patient outcomes based on molecular subtypes has been assessed in a head-to-head comparison with IHC and the Prosigna assay was shown to be more accurate.6 Errors in assigning subtype can significantly affect breast cancer treatment and outcomes (St. Gallen International expert concensus11).
All prognostic multigene assays achieve committee consensus.
The 50 gene assay (Prosigna) is recommended for use by the NCCN® Guidelines :
The Prosigna assay is recommended in conjunction with other clinicopathological variables to guide decisions on adjuvant systemic therapy for women with node-negative early-stage breast cancer.
Prosigna is recognized with level I evidence, at parity with other established gene expression assays. PAM50-based molecular subtypes are recommended for use to predict the benefit of neoadjuvant chemotherapy.
Prosigna has received the highest support among all tests for prognosis in years 1–5 for ER+, HER2-, pN0 and pN+ EBC.
Prosigna had the greatest level of support among all tests for late recurrence (years 5–10).
PAM50 subtypes are recognized as predictive by this body.
Prosigna is recommended as an option for guiding adjuvant chemotherapy decisions for people with HR+/HER2- lymph node (LN)-negative early breast cancer only if:
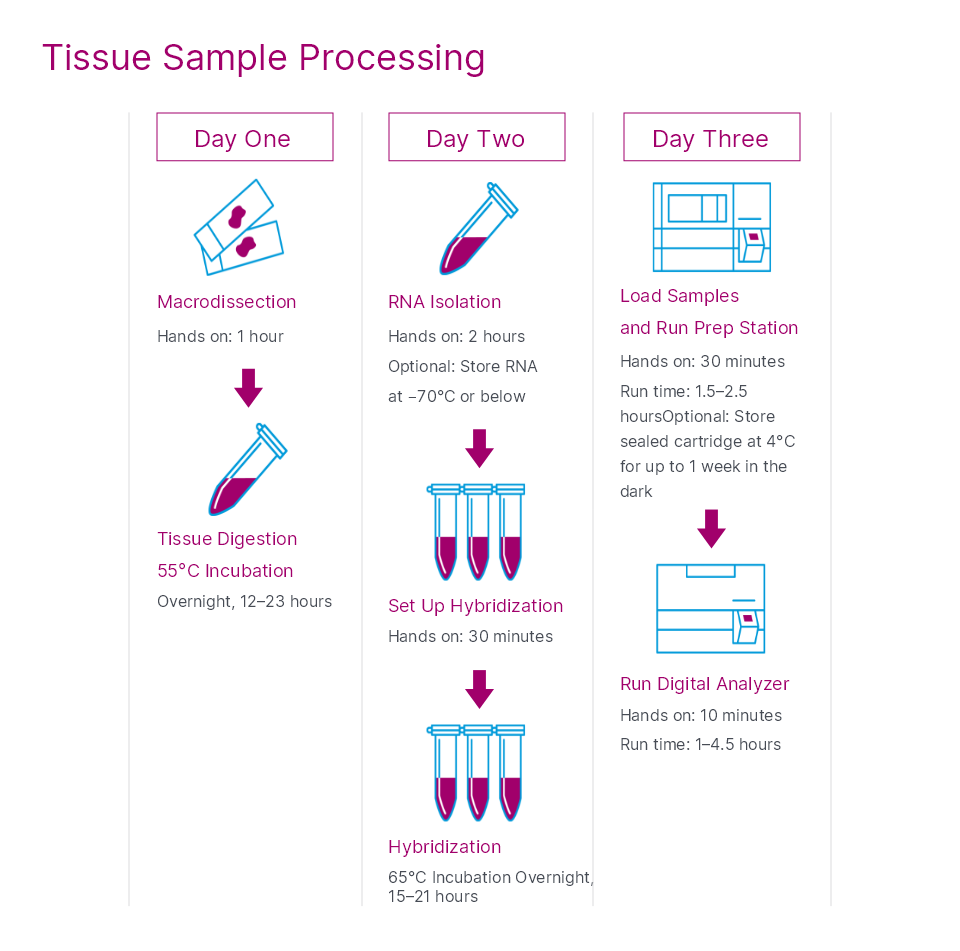
Prosigna® Breast Cancer Prognostic Gene Signature Assay (Prosigna assay) for use on the nCounter® Analysis System is CE-marked for in vitro diagnostic use in prognosis and surgical resection. Please refer to region specific Package Inserts for the respective product claims.
Prosigna® in conjunction with the nCounter® Analysis System is CE marked for in vitro diagnostic use in post-menopausal women with Hormone Receptor-Positive (HR+), lymph node-negative, Stage I or II breast cancer and post-menopausal women with Hormone Receptor-Positive (HR+), lymph node positive (1–3 positive nodes), Stage II & IIIA breast cancer to be treated with adjuvant endocrine therapy. See Package Insert for further details at prosigna.com. ©2021 Veracyte, Inc. Prosigna and the Prosigna logo are trademarks and/or registered trademarks of Veracyte, Inc. in various jurisdictions.
ASCO and ESMO are trademarks of the American Society of Clinical Oncology and European Society for Medical Oncology. National Institute for Health and Care Excellence (NICE), St Gallen International Consensus Panel, ASCO and ESMO do not endorse any product or therapy.
* Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.5.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed [December 5, 2023]. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. NCCN=National Comprehensive Cancer Network® (NCCN®)

Surgery: Breast conserving surgery (BCS) and sentinel lymph node biopsy (SLNB)
Pathology: Invasive ductal cancer (IDC) grade III 15 mm
Relatively small tumor
Subtype: Luminal A
Prosigna Risk of Recurrence (ROR) Score: 25
Prosigna Risk Group: 4%, low risk

Surgery: Breast conserving surgery (BCS) and sentinel lymph node biopsy (SLNB)
Pathology: Invasive ductal cancer (IDC) grade III 17 mm
Relatively small tumor with unfavorable biology
Subtype: Luminal B
Prosigna Risk of Recurrence (ROR) Score: 70
Prosigna Risk Group: 21%, high risk

Surgery: Breast conserving surgery (BCS) and sentinel lymph node biopsy (SLNB)
Pathology: Invasive ductal cancer (IDC) grade III 15 mm
Moderately sized Tumor
Subtype: Luminal A
Prosigna Risk of Recurrence (ROR) Score: 8
Prosigna Risk Group: 5%, low risk

To order the Prosigna Prognostic Gene Signature Assay please contact Veracyte at orders@veracyte.com
To re-order the Prosigna Prognostic Gene Signature Assay Kit please contact Veracyte at orders@veracyte.com

To order the nCounter® Analysis System for your site please contact Veracyte at orders@veracyte.com
Contact the Prosigna team
References: 1. Alexandre M, et al. Cancer Manag Res. 2019;11:10353–10373. 2. Sestak I, et al. JAMA Oncol. 2018;4(4):545–553. 3. Lænkholm AVet al. J Clin Oncol. 018;10;36(8):735-740. 4. Costa et al. J Clin Oncol. 2018;10;36(8):725-727. 5. Gnant M, et al. Ann Oncol. 2014;25(2):339-45.6. Dowsett M, et al. J Clin Oncol. 2013;31(22):2783-2790. 7. Prosigna [CE-IVD Package Insert] South San Francisco, CA: Veracyte, Inc; 2022-05 LB-0032-01. 8. Parker JS, et al. J Clin Oncol. 2009;27(8):1160-1167. 9. Perou M, et al. Nature 2000; 406(6796):747–752 10. Cejalvo JM et al.Cancer Treat Rev. 2018 Jun;67:63-70. 11. Goldhirsch A, at al. Annals of Oncology 2013;24: 2206–2223.